Any substance that can diminish the velocity of an enzyme-catalyzed reaction is called an inhibitor and the process is known as inhibition. There are two major types of enzyme inhibition, Irreversible and Reversible.
Irreversible Inhibition
The type of inhibition that cannot be reversed by increasing substrate concentration or removing the remaining free inhibitor is called Irreversible inhibition.
Example: Diisopropyl fluorophosphate (DFP) inhibits the enzyme acetyl cholinesterase, important in the transmission of nerve impulses. Acetyl cholinesterase catalyzes the hydrolysis of Acetylcholine (to acetic acid and choline) a neurotransmitter substance functioning in certain portions of the nervous system
DEP also inhibits enzymes such as trypsin, chymotrypsin, elastase, and phosphoglucomutase. Similarly, organophosphorus compounds like malathion and parathion, which are pesticides, inhibit acetylcholinesterase in the same manner as DFP.
Example: Inhibition of triose phosphate dehydrogenate by iodoacetate which block the activity of the enzyme.
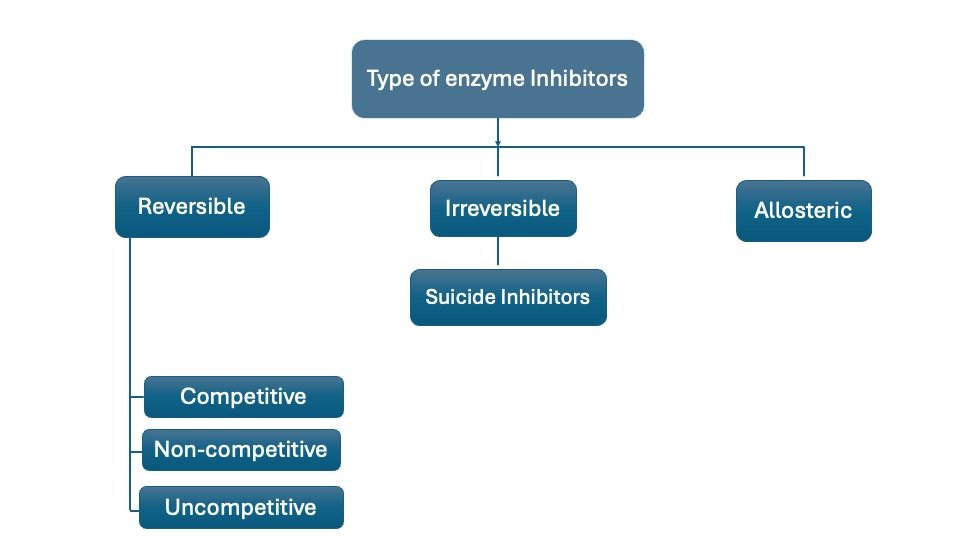
Reversible Inhibition
This type of inhibition can be Competitive, Non-competitive, and uncompetitive
A. Competitive Inhibition: This type of inhibition occurs when the inhibitor binds reversibly to the same site that the substrate would normally occupy, therefore, competes with the substrate for that site.
In competitive inhibition the inhibitor and substrate compete for the same active site on the enzyme because of similarity in structure. The enzyme substrate complex will be broken dawn to products (E+S ES E+P) whereas enzyme inhibitor complex; (EI) will not be broken down to products.
A classic example is Malonate that competes with succinate and inhibits the action of succinate dehydrogenase to produce fumarate in the Krebs cycle. The enzyme can be also inhibited by oxalate and glutamate because of the similarity of this substance with succinate.
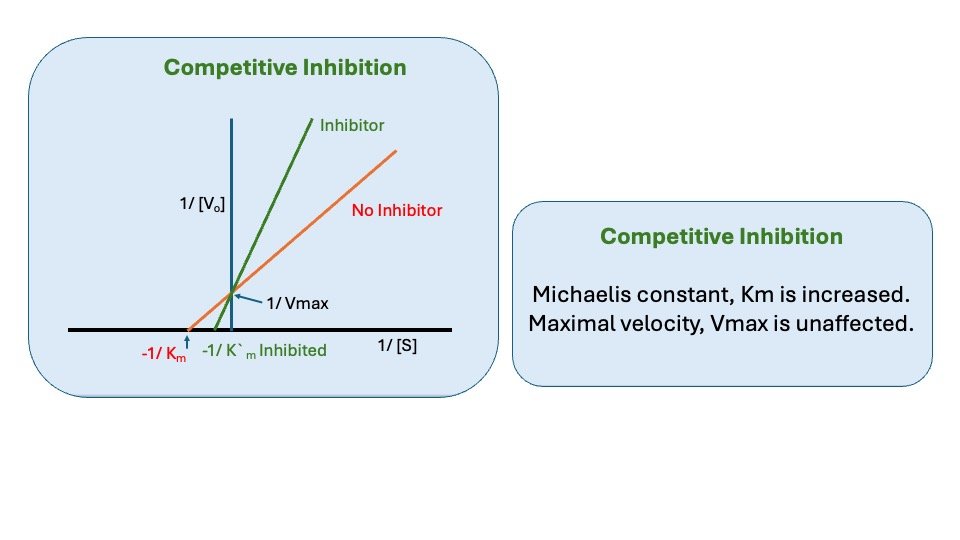
Figure: Competitive inhibition
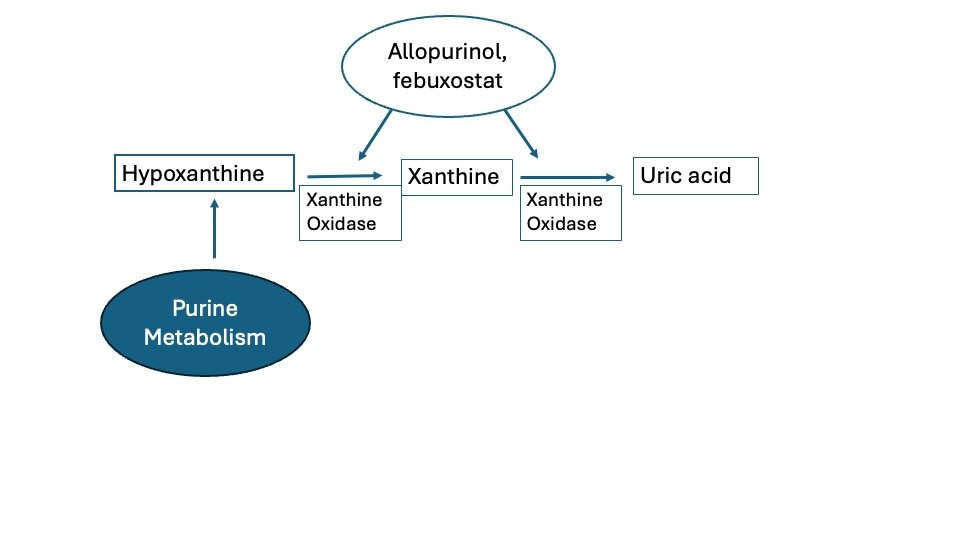
Another example, Allopurinol used for the treatment of Gout, Allopurinol Inhibits Xanthine oxidase by competing with Uric acid precursors for the active site on the enzyme. This competition blocks the conversion of these precursors, and of hypoxanthine and xanthine, to uric acid and result in lower serum urate levels.
Note: Inhibition of Enzyme Catalyzed Reactions:
To circumvent the complexities of curvilinear plots in enzyme-catalyzed reactions, and ease of understanding, biochemists Lineweaver and Burk devised an analysis method for enzyme kinetics that involves a rearrangement of the Michaelis-Menten equation.
[1/v] = [Km (1)/ Vmax[S] + (1)/[Vmax]
Plots of 1/v versus 1/[S] yield straight lines having a slope of Km/Vmax and an intercept on the ordinate at 1/Vmax. An alternative linear transformation of the Michaelis-Menten equation is the Eadie-Hofstee transformation:
v/[S] = -v [1/Km] + [Vmax/Km]
and when v/[S] is plotted on the y-axis versus v on the x-axis, the result is a linear plot with a slope of -1/Km and the value Vmax/Km as the intercept on the y- axis and Vmax as the intercept on the x-axis.
Both the Lineweaver-Burk and Eadie-Hofstee transformation of the Michaelis-Menton equation are useful in the analysis of enzyme inhibition. Since most clinical drug therapy is based on inhibiting the activity of enzymes, analysis of enzyme reactions using the tools described above has been fundamental to the modern design of pharmaceuticals
Effect of Competitive inhibitors
1. Effect on Vmax: The effect of a competitive inhibitor is reversed by increasing [s]. at a sufficiently high substrate concentration, the reaction velocity reaches the Vmax. observed in the absence of inhibitor.
2. Effect on Km: A competitive inhibitor increases the apparent Km for a given substrate. This means that in the presence of a competitive inhibitor more substrate is needed to achieve ½ Vmax.
B. Non-Competitive Inhibition
In non-competitive inhibition the inhibitor binds at different site rather than the substrate-binding site. When the inhibitor binds at this site there will be a change in conformation of the enzyme molecules, which leads to the reversible inactivation of the catalytic site. Non-competitive inhibitors bind reversibly either to the free-enzyme or the ES complex to form the inactive complexes EI and ESI (Enzyme substrate Inhibition)
The most important non-competitive inhibitors are naturally occurring metabolic intermediates that can combine reversibly with specific sites on certain regulatory enzymes, that changes the activity of their catalytic sites.
An Example: is the inhibition of L-threonine dehydratase by L-isoleucine.
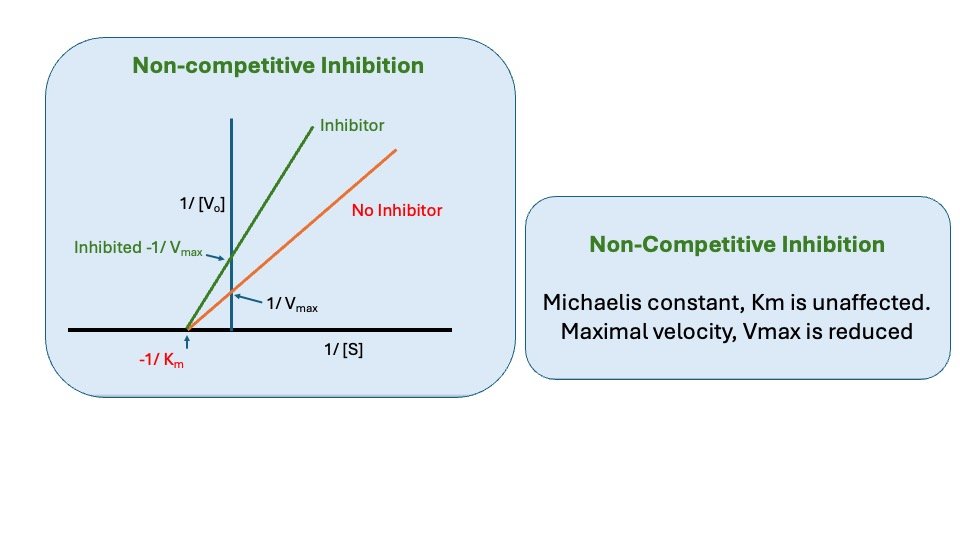
Figure: Noncompetitive inhibition
*Such type of Enzyme is called Allosteric Enzyme, which has a specific sites or allosteric site other than the substrate-binding site.
1. Effect on Vmax: Non-Competitive inhibition cannot be overcome by increasing the concentration of substrate. Thus, non-competitive inhibitors decrease the Vmas of the reaction.
2. Effect on Km: Non-competitive inhibitors do not interfere with the binding of substrate to enzyme. Thus, the enzyme shows the same Km in the presence or absence of the non- competitive inhibitor.
C. Uncompetitive Inhibition
Uncompetitive Inhibitor binds only to ES complex at locations other than the catalytic site. Substrate binding modifies enzyme structure, making inhibitor-binding site available. Inhibition cannot be reversed by substrate. In this case apparent Vmax and Km decreased.
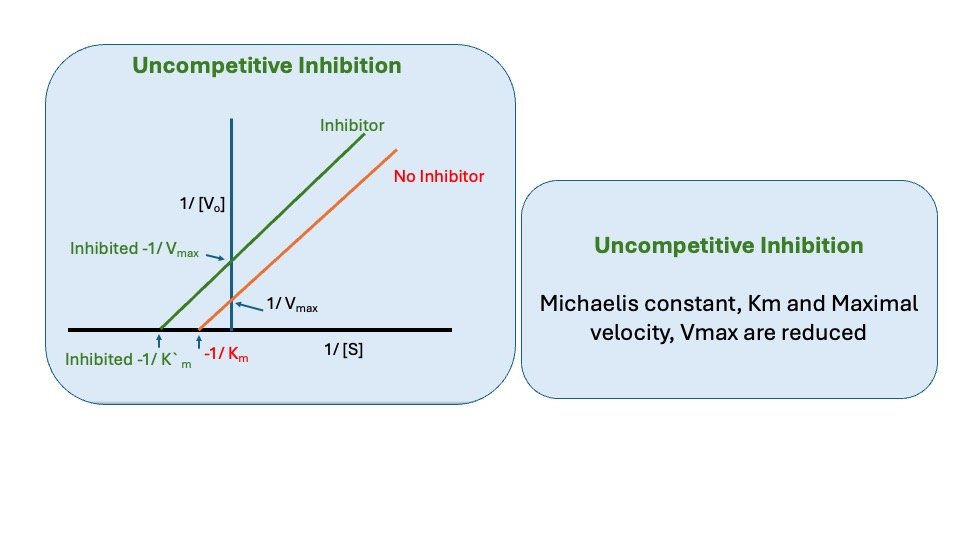
Figure: Uncompetitive inhibition
Regulation of enzyme activity
There are several means by which the activity of a particular enzyme is specifically regulated.
A. Irreversible covalent Activation / Zymogen activation
Proenzymes or zymogens are inactive forms of enzymes that are produced.
Specific peptide bonds are hydrolyzed at the site of action, either enzymatically or by PH changes, to transform it into active form, e.g. pepsinogen-pepsin, trypsinogen-trypsin, plasminogen-plasmin. Activation of enzyme from proenzyme is irreversible process.
B. Reversible Covalent Modification
Certain enzymes are reversibly activated and inactivated as required by the addition or removal of phosphate or adenylate. Muscle protein kinase uses ATP to phosphorylate phosphorylase kinase and glycogen synthetase.
C. Allosteric Modulation
In addition to simple enzymes, there is a family of enzymes that bind small, physiologically significant molecules other than active site and modify activity.
These enzymes are known as allosteric enzymes, and the small regulatory molecules that bind to them are called allosteric effectors. Allosteric effectors catalyze catalysis by binding to the enzyme at distinct allosteric sites far from the catalytic site and producing conformational changes that are communicated through the protein’s bulk to the catalytically active site (s). Effectors are characterized by their ability to bind to enzymes and modify the catalytic properties of the enzyme’s active site. Positive effectors increase catalytic activity, while negative effectors decrease or inhibit the activity of the catalyst.
There are two ways that enzymatic activity can be altered by effectors: the Vmax can be increased or decreased, or the Km can be raised or lowered.
D. Feedback inhibition
In allosteric regulation in which end products inhibit the activity of the enzyme is called” feedback inhibition”.
Isoleucine high conc. typically inhibits conversion of A to B.
This involves not simple backing up of intermediates but the activity of D to bind to and inhibit E1. D thus acts as negative allosteric affector or feedback inhibitor of E1.
The kinetics of feedback inhibition cay be competitive, mixed, etc. It is the commonest way of regulation of a biosynthetic pathway. Feedback regulation generally occurs at the earliest functionally irreversible step unique in the biosynthetic pathway.
ENZYMES IN CLINICAL DIAGNOSIS
- Measuring enzyme levels in blood is important for diagnosis.
- Enzymes in the blood often mean tissue or cell damage has occurred.
- Damaged cells release these enzymes into the blood.
- For example, testing for liver enzymes helps check for liver cell damage.
Plasma enzymes can be classified into two major groups
1. Those, relatively, small group of enzymes secreted into the plasma by certain organs (i.e. Enzymes those have function in plasma) For example: – the liver secretes zymogens of the enzymes involved in blood coagulation.
2. Those large enzyme species released from cells during normal cell turnover. These enzymes are normally intracellular and have no physiologic function in the plasma. In healthy individuals the levels of these enzymes are fairly constant and represent steady state in which the rate of release from cells into the plasma is balanced by an equal rate or removal from the plasma.
- Tissue-damaging diseases often lead to more enzymes being released into the blood.
- Enzyme activity in blood is commonly checked to diagnose issues in the heart, liver, muscles, and other tissues.
- Higher enzyme levels in blood usually mean more tissue damage.
- The degree of enzyme increase helps with diagnosis and predicting recovery.
- Measuring enzyme levels from specific tissues provides useful information about diseases in those tissues.
1. Lipase: It is an enzyme catalyzing the hydrolysis of fats. It is secreted by pancreas and Liver. The plasma lipase level may be low in liver disease, Vitamin A deficiency, some malignancies, and diabetes mellitus. It may be elevated in acute pancreatitis and pancreatic carcinoma.
2. Alpha-amylase is an enzyme responsible for breaking down dietary starch and glycogen into maltose. It is found in pancreatic juice, saliva, liver, fallopian tubes, and muscles. This enzyme is also excreted in urine. Amylase level measurements are primarily used in diagnosing acute pancreatitis. Plasma amylase levels may decrease in liver disease and increase in conditions such as high intestinal obstruction, mumps, acute pancreatitis, and diabetes.
3. Trypsin is secreted by pancreas. Elevated levels of trypsin in plasma occur during acute pancreatic disease.
4. Alkaline phosphatases (ALP) are enzymes that break down phosphate compounds in an alkaline environment.
- ALP is found in bone, liver, kidneys, intestines, lactating mammary glands, and placenta.
- In bone, ALP is present in osteoblasts and supports normal bone function.
- ALP levels can rise in conditions like:
- Rickets and osteomalacia (bone disorders)
- Hyperparathyroidism
- Paget’s disease of bone
- Obstructive jaundice
- Metastatic cancer
ALP levels may also increase in congestive heart failure due to liver injury.
5. Acid Phosphatase (ACP), which catalyzes the hydrolysis of various phosphate esters at an acidic pH, is present in the prostate, liver, red blood cells, platelets, and bone. Its levels may be elevated in cases of metastatic prostatic carcinoma.
6. Transaminases: Two transaminases are of clinical interest.
a. Aspartate Transaminase, AST (Serum Glutamate Oxaloacetate Transaminase, SGOT) catalyzes the transfer of the amino group of aspartic acid to a-ketoglutarate forming glutamate and oxaloacetate. AST or SGOT is widely distributed, with high concentration, in the heart, liver, skeletal muscle, kidney and erythrocytes, and damage to any of these tissues may cause raised levels.
b. Alanine transaminase, ALT (Serum Glutamate Pyruvate Transaminase, SGPT) Transfer the amino group of alanine to a-ketoglutarate, forming glutamate and pyruvate. It is present in high concentration in liver and to a lesser extent in skeletal muscle, kidney and heart.
SGPT and SGOT levels help diagnose liver and myocardial damage. In liver damage, SGPT rises more than SGOT, while in myocardial infarction, SGOT increases with little change in SGPT.
7. Lactate Dehydrogenase (LDH): It catalyzes the reversible interconversion of lactate and pyruvate. It is widely distributed with high concentrations in the heart, skeletal muscle, liver, kidney, brain and erythrocytes. The enzyme is increased in plasma in myocardial infarction, acute leukemias, and in acute hepatitis. Estimation of it isoenzymes is more useful in clinical diagnosis to differentiate hepatic disease and myocardial infarction (also see Isoenzyme section).
8. Creatine kinase (CK) or creatine phosphokinase (CPK), CPK is found in heart muscle brain and skeletal muscle. Measurement of serum creatine phosphokinase activity is of value in the diagnosis of disorders affecting skeletal and cardiac muscle. The level of CPK in plasma highly increased in myocardial infarction (also see Isoenzyme section).
Enzymes are recognized as markers of cellular damage. The concentration of enzymes in the plasma is utilized to investigate diseases of the liver, heart, skeletal muscle, biliary tract, and more.
Classification of diagnostically important enzymes:
- Liver, cardiac and skeletal enzymes
- Biliary tract enzymes
- Digestive enzymes of pancreatic origin
Liver, cardiac and skeletal enzymes:- Enzymes in this category include:
- Aminotransferases or transaminase
- Increases plasma Aspartate aminotransferase (AST or SGOT) level and increased plasma alanine aminotransferase (ALT or SGPT) level indicates liver diseases.
- Increased AST level occurs after myocardial infraction as heart muscle contains relatively high concentration of AST.
- AST activity levels are increased in muscular dystrophy and dermatomyositis.
- Moderate increase of both AST and ALT have been observed after the intake of alcohol, or after taking various drugs such as salicylates ampicillin etc.
- Alkaline phosphatase:- Plasma ALP level are particularly used in the investigation of bone diseases and hepatobiliary diseases.
Biliary tract enzymes:- Enzymes included in this category are
- 5’-Nucleotidase (nucleotide phosphate NTP)- Increased level of serum NTP occur in hepatobiliary diseases in which there is obstruction in the secretion of bile.
- Gamma-glutamyl transferase GGT– Increased GGT levels indicate liver diseases and are used to investigate obstructive jaundice, cholangitis, and cholecystitis.
Digestive enzymes of pancreatic origin:- The digestive enzymes are investigated for the diagnosis of pancreatic diseases
- Alpha-amylase:- Assays of amylase in the serum and urine are primarily used for the diagnosis of pancreatic diseases like pancreatitis, cholecystitis, tumors etc.
- Trypsin:- The measurement of serum trypsin can be utilized to screen for cystic fibrosis within the first six weeks of life. Additionally, it is applied in assessing pancreatic ductules.
- Chymotrypsin:- Similar to amylase and trypsin, an increase in serum chymotrypsin levels, reaching eight times the normal threshold, indicates the presence of renal failure.
In short
Enzymes are crucial biological catalysts that accelerate chemical reactions by lowering activation energy. They are predominantly proteins and exhibit high specificity and catalytic efficiency.
Types of Enzymes:
- Simple Enzymes: Composed solely of proteins (e.g., Pancreatic Ribonuclease).
- Holoenzymes: Consist of a protein part (apoenzyme) and a non-protein component (cofactor).
Cofactors can be organic (coenzymes) or inorganic ions (activators like Fe²⁺, Zn²⁺).
Catalytic Properties:
- Active Site: The unique region where substrates bind and reactions occur.
- Turnover Number (kcat): Measures the number of substrate molecules converted per enzyme per second.
- Specificity: Enzymes are selective for substrates, showing absolute, stereo, bond, or geometric specificity.
Enzyme Regulation:
- Zymogens: Inactive enzyme precursors activated when needed (e.g., Pepsinogen to Pepsin).
- Allosteric Regulation: Enzymes modulated by molecules binding at sites other than the active site.
Enzyme Kinetics:
- Michaelis-Menten Equation: Describes the rate of enzymatic reactions, with Km indicating substrate affinity.
Enzyme Inhibition:
- Competitive Inhibition: Inhibitor competes with substrate for the active site.
- Non-Competitive Inhibition: Inhibitor binds to a different site, altering enzyme activity.
Clinical Relevance:
Enzyme levels in blood can indicate tissue damage (e.g., elevated CK-MB suggests myocardial damage).
Types of Enzymes and Reactions:
- Oxidoreductases: Catalyze redox reactions (e.g., Lactate dehydrogenase).
- Transferases: Transfer groups between molecules (e.g., Hexokinase).
- Hydrolases: Break bonds using water (e.g., β-Galactosidase).
- Lyases: Remove groups without hydrolysis, forming double bonds (e.g., Fumarase).
- Isomerases: Catalyze isomerization changes.
- Ligases: Join two molecules with energy input (e.g., DNA Ligase).
- Translocases: Facilitate ion/molecule movement across membranes.
Enzymes are essential for efficient biochemical processes, and understanding their function and regulation is vital in fields like medicine and biochemistry.
Reference book
- Harper’s Illustrated Biochemistry, Thirty-Second Edition
- Textbook of Biochemistry with Clinical Correlations Hardcover – Illustrated, 22 January 2010 by Thomas M. Devlin
- Lehninger Principles of Biochemistry: 6th Edition
- Lippincott’s Illustrated Reviews – Biochemistry South
Multiple Choice Question
1. Which of the following best describes enzyme inhibition?
A) Acceleration of enzyme activity
B) Temporary or permanent reduction of enzyme activity
C) Complete destruction of enzyme structure
D) None of the above
Answer: B
2. Competitive inhibitors primarily bind to:
A) The active site of the enzyme
B) The allosteric site
C) The substrate
D) A coenzyme
Answer: A
3. In competitive inhibition, the inhibitor:
A) Changes the enzyme’s shape
B) Binds to a different site than the substrate
C) Competes with the substrate for the active site
D) Activates the enzyme
Answer: C
4. Which of the following is NOT a type of enzyme inhibition?
A) Competitive inhibition
B) Non-competitive inhibition
C) Allosteric activation
D) Uncompetitive inhibition
Answer: C
5. In non-competitive inhibition, the inhibitor binds to:
A) The active site
B) The substrate
C) An allosteric site
D) The product
Answer: C
6. Uncompetitive inhibitors bind to:
A) The free enzyme only
B) The enzyme-substrate complex
C) The active site alone
D) Both the enzyme and enzyme-substrate complex
Answer: B
7. In competitive inhibition, the Vmax of the enzyme:
A) Increases
B) Decreases
C) Remains unchanged
D) Varies with substrate concentration
Answer: C
8. Which of the following changes in a Lineweaver-Burk plot indicates competitive inhibition?
A) Parallel lines
B) Lines intersecting at the y-axis
C) Lines intersecting at the x-axis
D) No changes
Answer: B
9. Which of the following is TRUE for a non-competitive inhibitor?
A) It only binds to the free enzyme.
B) It reduces the maximum reaction rate (Vmax).
C) It only binds to the active site.
D) It changes the Km of the enzyme.
Answer: B
10. Allosteric inhibition is also known as:
A) Competitive inhibition
B) Feedback inhibition
C) Enzyme denaturation
D) Irreversible inhibition
Answer: B
11. Which type of inhibition can be reversed by increasing the concentration of the substrate?
A) Competitive
B) Non-competitive
C) Uncompetitive
D) Irreversible
Answer: A
12. Irreversible inhibitors typically:
A) Form strong covalent bonds with the enzyme
B) Can be removed by dialysis
C) Bind weakly to the enzyme
D) None of the above
Answer: A
13. Which of the following is a common example of a competitive inhibitor?
A) Cyanide
B) Aspirin
C) Malonate
D) None of the above
Answer: C
14. Which statement about uncompetitive inhibition is correct?
A) The inhibitor only binds to the free enzyme.
B) Both Km and Vmax are affected.
C) It binds at the active site.
D) Km remains unchanged.
Answer: B
15. In feedback inhibition, the end product of a metabolic pathway:
A) Activates the first enzyme in the pathway
B) Inhibits the first enzyme in the pathway
C) Binds only to the substrate
D) Has no effect on enzyme activity
Answer: B
16. A Lineweaver-Burk plot of an enzyme with a non-competitive inhibitor shows:
A) No change in Vmax
B) No change in Km
C) A decrease in Km
D) A decrease in Vmax
Answer: D
17. Which of the following inhibitors is known for binding permanently to enzymes, causing irreversible inhibition?
A) Penicillin
B) Malonate
C) Carbon monoxide
D) EDTA
Answer: A
18. Which of the following is NOT affected by non-competitive inhibition?
A) Km
B) Vmax
C) Enzyme turnover number
D) Active site structure
Answer: A
19. Enzyme inhibitors that structurally resemble the substrate are generally:
A) Uncompetitive inhibitors
B) Non-competitive inhibitors
C) Competitive inhibitors
D) Irreversible inhibitors
Answer: C
20. Which of the following statements is true regarding irreversible inhibition?
A) The inhibitor can be removed by increasing substrate concentration.
B) It typically forms covalent bonds with the enzyme.
C) The inhibitor competes with the substrate.
D) It affects only the Km value.
Answer: B
21. A substance that slows enzyme activity by binding to a site other than the active site is called a(n):
A) Competitive inhibitor
B) Uncompetitive inhibitor
C) Non-competitive inhibitor
D) Allosteric activator
Answer: C
22. Which of the following inhibitors decreases both Vmax and Km?
A) Competitive inhibitor
B) Non-competitive inhibitor
C) Uncompetitive inhibitor
D) Irreversible inhibitor
Answer: C
23. The inhibition of succinate dehydrogenase by malonate is an example of:
A) Non-competitive inhibition
B) Irreversible inhibition
C) Competitive inhibition
D) Uncompetitive inhibition
Answer: C
24. Which enzyme inhibition can often be reversed by removing the inhibitor?
A) Irreversible inhibition
B) Reversible inhibition
C) Non-reversible inhibition
D) Permanent inhibition
Answer: B
25. Which enzyme inhibition method is commonly used as a regulation mechanism in metabolic pathways?
A) Competitive inhibition
B) Non-competitive inhibition
C) Feedback inhibition
D) Irreversible inhibition
Answer: C