Enzymes are biocatalysts present in cells that speed up biochemical reactions without getting itself destroyed in the reaction.Enzymes accelerate the rate of biochemical reactions by lowering the activation energy (the amount of energy needed to start the reaction).
An enzyme is a protein (99%) or RNA (1%) produced by living cells, which is highly specific and highly catalytic to its substrates. Enzymes are crucial macromolecular biological catalysts. Because of enzyme activity, chemical reactions within organisms can proceed efficiently and with specificity under mild conditions.
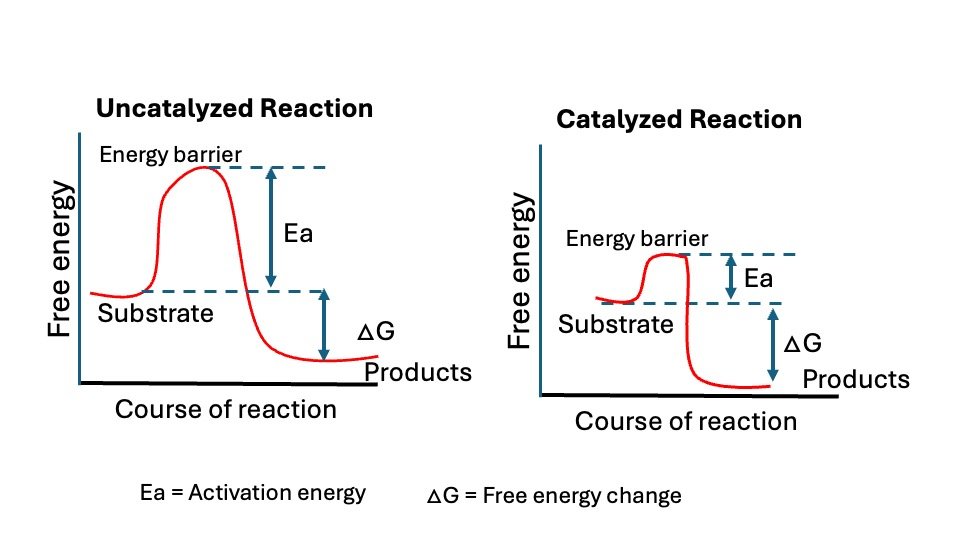
Figure: Graph shown without and with an enzyme catalyzed reaction. Ea= activation energy and Delta G = Free energy change.
General Properties
Most enzymes are mostly protein in nature. Depending on the presence and absence of a non-protein component with the enzymes can exist as, simple enzyme or holoenzyme.
1. Simple enzyme: It is made up of only protein molecules not bound to any non- proteins. Example: Pancreatic Ribonuclease.
2. Holo enzyme is made up protein and non-protein component.
- Apoenzyme: The protein component of this holoenzymes is called apoenzyme
- Cofactor: The non-protein component of the holoenzyme is called a cofactor.
If the cofactor is an organic compound, it’s referred to as a coenzyme, and if it’s an inorganic group, it’s known as an activator. (Fe2+, Mn2+, or Zn2+ ions).
When a cofactor is tightly bound to an apoenzyme and cannot be easily removed without damaging the enzyme, it is referred to as a prosthetic group.
Coenzymes are vitamin derivatives that are essential for the functioning of various enzymes, enabling them to catalyze reactions. A single molecule of coenzyme can facilitate the conversion of many substrate molecules when paired with an enzyme.
- Coenzyme accepts a particular group removed from the substrate or donates a particular group to the substrate (converted it into product).
- Sometimes coenzymes are called co-substrate because the changes that take place in substrates are complimentary to the changes in coenzymes.
- The coenzyme may participate in forming an intermediate enzyme-substrate complex. Example: NAD, FAD, Coenzyme A
Metal ion in enzymes: Many enzymes require metal ions like Ca2+, K+, Mg2+, Fe2+, Cu2+, Zn2+, Mn2+ and Co2+ for their activity.
| S.No. | Enzyme | Metal Ions |
| 1 | Ascorbic acid oxidase: | Uses copper |
| 2 | Alcohol dehydrogenase: | Uses zinc |
| 3 | Glutamine synthetase: | Uses magnesium |
| 4 | Isocitrate lyase: | Uses magnesium |
| 5 | Xanthine oxidase: | Uses molybdenum |
| 6 | Glutathione peroxidase: | Uses selenium |
Metal ions promote enzyme action by
a. Maintaining or producing the active structural conformation of the enzyme (e.g. Magnesium ions activate glutamine synthase)
b. Promoting the formation of the enzyme-substrate complex (Example: Zinc ion in carboxypeptidase A)
c. Acting as electron donors or acceptors (Example: Fe-S proteins and cytochromes in ETC)
d. Causing distortions in the substrate or the enzyme Example: phosphotransferases.
Properties of Enzyme
A. Active site
Enzyme molecules possess a unique pocket or cleft known as the active site. This site comprises amino acid chains that facilitate the formation of a three-dimensional surface, which is also complementary to the substrate.
The active site of an enzyme is the part of the enzyme where substrate molecules bind and a chemical reaction takes place. The active site is made up of amino acid residues that establish temporary bonds with the substrate (binding site) as well as residues that catalyse that substrate’s reaction (catalytic site).
The active site binds the substrate, forming an enzyme-substrate (ES) complex. ES is converted to enzyme-product (EP); which subsequently dissociates to enzyme and product. Every enzyme is believed to have one or more active sites where the substrate can bind.
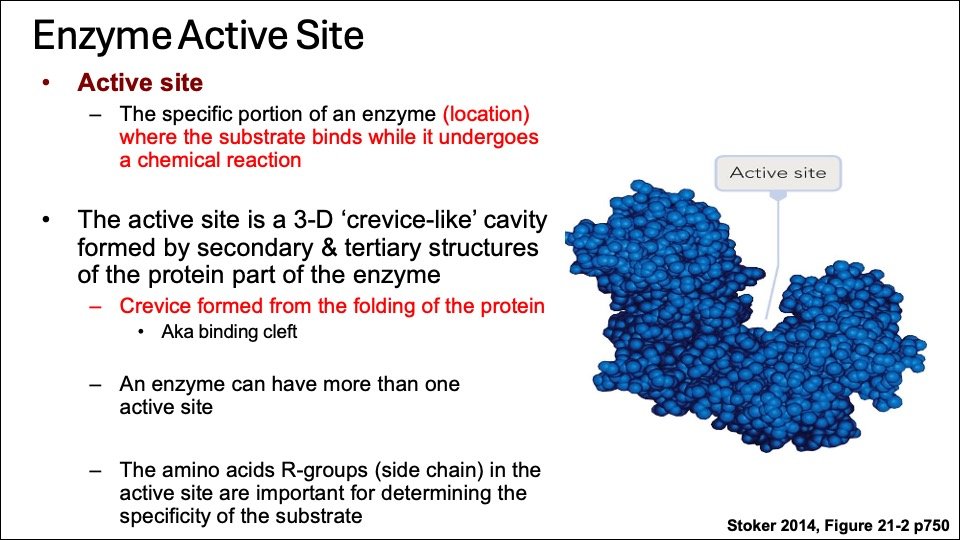
Figure: Enzyme 3D structure, Binding site (Blue) and Catalytic site (red) Courtesy : Stocker 2014
The active site of the enzyme may contain free hydroxyl group of serine, phenolic (hydroxyl) group of tyrosine, SH-thiol (Sulfhydryl) group of cysteine or imidazole group of histidine to interact with there substrates. Additionally, the active site, has catalytic site, which is situated near the binding site on the enzyme molecule.
B. Catalytic efficiency/ Enzyme turnover number (kcat)
Enzyme-catalyzed reactions are 1,000 to 100 million times faster than uncatalyzed reactions. Generally, a single enzyme molecule can transform between 100 and 1,000 substrate molecules into product molecules every second.
Enzyme turnover number, or kcat, indicates the amount of substrate converted to product per active site per unit time when the enzyme is saturated.This value is derived from the maximum velocity, with chymotrypsin's kcat being 100 per second.
- Catalase has the highest turnover rate of any enzyme, converting over 2.8 million hydrogen peroxide molecules into water and oxygen each second.
- Carbonic anhydrase facilitates the conversion of CO2 into bicarbonate at an impressive rate of 600,000 molecules per second.
C. Specificity
Enzymes are specific for their substrate. Specificity of enzymes are divided into:
a. Absolute specificity: This shows that one enzyme acts on a specific substrate; for instance, urease catalyzes urea hydrolysis but not thiourea.
b. Stereo specificity: Certain enzymes are specific to one isomer of a molecule. For example, glucose oxidase catalyzes only the oxidation of β-D-glucose, while arginase hydrolyzes L-arginine exclusively. Likewise, maltase catalyzes the hydrolysis of α-glycosides but not β-glycosides.
c. Bond Specificity: Enzymes that target specific bonds, such as ester, peptide, or glycosidic linkages, belong to this category.
Examples: Esterases– acts on ester bonds, Peptidases-acts on peptide bonds, Glycosidases– acts on glycosidic bonds.
d. Geometrical specificity: Enzymes demonstrate a preference for either cis or trans isomers. For example, fumarase assists in the conversion between fumarate and malate.
D. Regulation
Enzyme activity can be regulated; enzymes can be activated or inhibited to ensure that the rate of product formation meets the cell’s requirements.
Zymogens (inactive form of enzyme) Certain enzymes are synthesized in an inactive form and can be activated as needed. Zymogens, also known as proenzymes, are such enzymes. This category includes numerous digestive enzymes and those involved in blood clotting.
Examples: Pepsinogen: This zymogen originates from gastric juice. Upon necessity, pepsinogen is converted into pepsin.
Trypsinogen – This zymogen, present in pancreatic juice, is converted into trypsin when needed. The activation process is facilitated by certain ions or other proteolytic enzymes.
Note: Zymogen forms of enzymes a protective mechanism to prevent auto digestion of tissue producing the digestive enzymes and to prevent intravascular coagulation of blood.
Enzyme Units • Expressed in terms of the activity
• One International Unit (IU) – One IU defined as the enzyme activity that converts one micromole of substrate to products per minute under optimal conditions and defined temperature, expressed as IU/L.
• Katal– amount of enzyme catalyzing the conversion of 1 mole of substrate to product in 1 second. Because this is such a large unit for most enzymatic reactions, the nanokatal (nkat) is used in practice.
Isoenzymes (Isozymes)
Isoenzymes (or isozymes) are a group of enzymes that catalyze the same reaction but have different enzyme forms and catalytic efficiencies. Isozymes are usually distinguished by their electrophoretic mobilities and amino acid compositions.
Salient Features:
- Multiple forms of enzymes that catalyze the same reaction but differ in their structure
- Encoded by different structural gene loci.
- Differ in physical properties like electrophoretic motility or resistance to heat inactivation.
- Antigenically distinct
- Differ in catalytic properties like Km, Vmax.
- Shows tissue specific distribution.
Examples of Isoenzymes
LDH (Lactate dehydrogenase) LDH is an enzyme that helps convert lactic acid to pyruvic acid and vice versa.
- It exists in five forms (isoenzymes), each with four chains made of two subunits: M (Muscle) and H (Heart).
- LDH is widely found in the body, with high levels in the heart, liver, muscles, kidneys, and red blood cells, and lower levels in the lungs, smooth muscle, and brain.
- The five LDH isoenzymes are M4, M3H, M2H2, MH3, and H4, distributed differently across tissues.
- Electrophoresis can separate the five LDH isoenzymes:
- M4 (LDH-5) is mainly in the liver and skeletal muscle.
- H4 (LDH-1) is primarily in the heart.
- MH3 (LDH-2) is the main isoenzyme in normal blood serum.
- When muscle or liver disease increases total LDH, LDH-5 dominates.
- In a heart attack (myocardial infarction), LDH-1 becomes dominant, shifting from LDH-2 to LDH-1 in serum—this “LDH flip” indicates acute myocardial infarction.
| Isoenzyme | Composition | Present in | Elevated in |
| LDH1 | HHHH (H4) | Myocardium, RBC | Myocardial infarction |
| LDH2 | HHHM (H3M1) | Myocardium, RBC | |
| LDH3 | HHMM (H2M2) | Kidney, Skeletal muscle | |
| LDH4 | HMMM (H1M3) | Kidney, Skeletal muscle | |
| LDH5 | MMMM (M4) | Kidney, Skeletal muscle, Liver | Skeletal muscle and liver disease |
Types of polypeptide chain and tissue distribution and clinical significance
Creatine N phosphotransferase CK: Creatine kinase (CK) is an enzyme associated with ATP regeneration in contraction and transport systems, primarily functioning in muscle cells to store high-energy phosphocreatine.
CK is a dimer composed of two different subunits, B and M. The CK isoenzymes, designated MM, MB, and BB, are resolved by electrophoresis.
The BB isoenzyme is found in the brain and intestinal smooth muscle.
- BB isoenzyme is rarely present in blood serum, even with brain or intestinal disease.
- MM is the main CK isoenzyme in skeletal muscle and the heart (myocardium).
- Both skeletal muscle and the heart contain MB isoenzyme, but the MB/MM ratio differs:
- In skeletal muscle, MB is less than 1% of total CPK activity.
- In the heart, MB makes up about 30% of total CK activity.
- High CK-MB levels (≥6% of total CK) indicate heart damage, especially in heart attacks (acute myocardial infarction, or AMI).
After a heart attack, CK-MB levels:
- Rise in 4 to 8 hours.
- Peak in 12 to 24 hours.
- Return to normal within 48 to 72 hours.
Tracking CK-MB with LDH isoenzymes or troponins over 48 hours improves AMI diagnosis accuracy.
| CK-1 (CK-BB) | Found in Brain, Lung, prostate, Uterus, Placenta, thyroid | |
| CK-2 (CK-MB) | Predominates in : Cardiac muscle (30%) and Skeletal muscle (1%) | Elevated in Myocardial Infarction |
| Ck-3 (CK-MM) | Predominates in : Skeletal muscle (99%) and Cardiac muscle (70%) | Elevated in muscular dystrophy |
CK-BB levels are significantly elevated in patients with various carcinomas, suggesting it may serve as a tumor-associated marker. Common causes for CK-BB elevation include central nervous system damage, tumors, childbirth, and macro-CK, an enzyme–immunoglobulin complex.
Degenerative muscle disease or severe muscle injury increases total serum CK activity, however, MB isoenzyme content remains under 5%. An MB activity over 6% of total indicates a myocardial infarction (MI).
Hexokinase: (Hexokinase I-IV) The liver and pancreatic β cells have a different hexokinase isoform called Glucokinase
Characteristics of hexokinase IV and hexokinase I, II and III
Classification of Enzymes
The nomenclature of enzymes is derived from their substrates or the catalyzed chemical reactions, and “ase” is usually added as a suffix. Enzymes can be indexed with letters and numbers according to International Union of Biochemistry and Molecular Biology: the letter EC four numbers representing four elements. The first number represents enzymes that are classified according to the mechanism of enzymatic reaction.
- Classes
- Subclasses
- Sub-subclasses
- EC numbers (Enzyme commission)
Enzymes are classified based on catalytic reactions.
Each enzyme is characterized by a specific number comprising four digits separated by points. The four digits characterize class, sub-class, sub-sub-class, and serial number of a particular enzyme.
The International union of Biochemistry and Molecular Biology (IUBMB) established a system of nomenclature on which enzymes are divided into seven major classes, each with numerous subgroups. Enzymes are classified based on the reactions they catalyze.
| Enzyme class | Reaction type | Description | Examples |
| EC1 Oxidoreductases | Catalyze redox reaction and can be categorized into oxidase and reductase. | Oxidase, dehydrogenase | |
| EC2 Transferases | Catalyze the transfer or exchange of certain groups among some substrates | Transferase, Kinase | |
| EC3 Hydrolases | Accelerate the hydrolysis of substrates | Nuclease, protease, Lipase amylase | |
| EC4 Lyases | Promote the removal of a group from the substrate to leave a double bond reaction or catalyze its reverse reaction | decarboxylase, aldolase | |
| EC5 Isomerases | Facilitate the conversion of isomers, geometric isomers or optical isomers. | isomerase, mutase | |
| EC6 Ligases | Catalyze the synthesis of two molecular substrates into one molecular compound with the release energy | DNA ligases | |
| EC7 Translocases | Catalyze the movement of ions or molecules across membranes or their separation within membranes | Translocase |
Class I. Oxidoreductases: Enzymes catalyzing oxidation reduction reactions.
Example: Succinate dehydrogenase, Lactate dehydrogenase
Class II. Transferases: Enzymes catalyzing a transfer of a group other than hydrogen (methyl, acyl, amino or phosphate groups)
Example:
a. Hexokinase, Enzymes catalyzing transfer of phosphorus containing groups.
ATP: D-hexose-6 phosphotransferase (Hexokinase) ATP+D-Hexose ADP+D-hexose-6-phosphate
b. Acetyl-CoA: Choline D-acyltransferase (Choline Acyltransferase)
Class III. Hydrolases: Enzymes catalyzing hydrolysis of ester, ether, peptide, glycosyl, acid-anhydride, C-C, C-halide, or P-N-bonds by utilizing water.
Example: Enzymes action on glycosyl compounds
β-D- Galactoside galactohydrolase (β-Galactosidase)
β -D- Galactoside+H2O alcohol +D-Galactose
Class IV. Lyases: Enzymes that catalyze removal of groups from substances by mechanisms other than hydrolysis, leaving double bonds.
Example: Enzymes acting on C-C, C-O, C-N, C-S and C-halide bonds. Example: Carbon-Oxygen lyases. Example (Fumarase)
Class V. Isomerases: Includes all enzymes catalyzing interconversion of optical, geometric, or positional isomers.
Example: Triose phosphate isomerase: Enzymes catalyzing interconversion of aldose and ketoses.
D – Glucose-6- phosphate ketoisomerase (triosephosphate isomerase) D-Fructose-6-phosphate.
Class VI. Ligases or synthetases: Enzymes catalyzing the linking together of 2 compounds coupled to the breaking of a pyrophosphate bond in ATP or similar trinucleotides: GTP, UTP etc. included are enzymes catalyzing reactions forming C-O, C-S, C-N, and C-C bonds.
Example: Enzymes catalyzing formation of C-N-bonds,
L- Glutamine: ammonia ligase (ADP) [Glutamine Synthetase]
ATP + L-Glutamate + NH4 = ADP + orthophosphate + L-Glutamine
Example: DNA Ligase
Class VII. Translocases: Translocases are crucial enzymes that move ions or molecules across membranes.
For example, carnitine-acylcarnitine translocase transports carnitine-fatty acid complexes and free carnitine across the inner mitochondrial membrane, essential for fatty acid metabolism. ADP/ATP translocases facilitate the exchange of cytosolic ADP with mitochondrial ATP, also across the inner mitochondrial membrane.
MECHANISM OF ACTION OF ENZYMES
- Lock and Key model
- Emil Fischer proposed the Lock and Key model 1894.
- The lock and key model explains enzyme-substrate interaction.
- Proposed by Emil Fischer in 1894.
- The enzyme’s active site is shaped to fit its substrate, similar to a lock and key.
Overview of the concept:
- Specificity: The model suggests that enzymes and substrates have complementary shapes that fit together precisely, similar to a key and lock.
- Mechanism: In this model, the enzyme (the lock) has an active site with a specific shape. Only the substrate (the key) with the matching shape can bind to the enzyme’s active site, leading to a chemical reaction.
- Analogy: Think of it as a lock and key mechanism where only the correct key (substrate) can open the lock (enzyme), ensuring high specificity in biochemical reactions.
This model is fundamental in understanding enzyme specificity and catalysis, although it has been complemented by the induced fit model, which suggests that enzymes are more flexible and can adjust their shape to fit the substrate more closely.

Figure: Lock and Key Model of enzyme- substrate interactions
2. Induced Fit Model:
The induced fit model explains enzyme-substrate interactions, suggesting that enzyme shapes and active sites become complementary to substrates only after binding, a concept proposed by Daniel Koshland in 1958.
- Concept: The induced fit model suggests that an enzyme’s active site is flexible and adjusts its shape to fit the substrate, unlike the older “lock and key” model, which suggests a perfect fit.
- Mechanism: When a substrate binds to an enzyme, it causes a conformational change in the active site, improving the enzyme’s ability to align catalytic groups and enhance reaction catalysis.
- Analogy: Imagine a hand (substrate) fitting perfectly into a glove (enzyme), which adjusts its shape to match.
- Advantages: This model describes enzyme behaviors, including noncompetitive inhibition, where an inhibitor binds to an allosteric site, altering enzyme shape and preventing catalysis.
Key Points
- Dynamic Interaction: Both the enzyme and substrate undergo conformational changes until the substrate is fully bound.
- Catalytic Efficiency: The induced fit enhances the enzyme’s ability to lower the activation energy of the reaction, increasing the reaction rate.
- Specificity and Regulation: This model accounts for the high specificity of enzyme-substrate interactions and the regulatory mechanisms of enzyme activity.
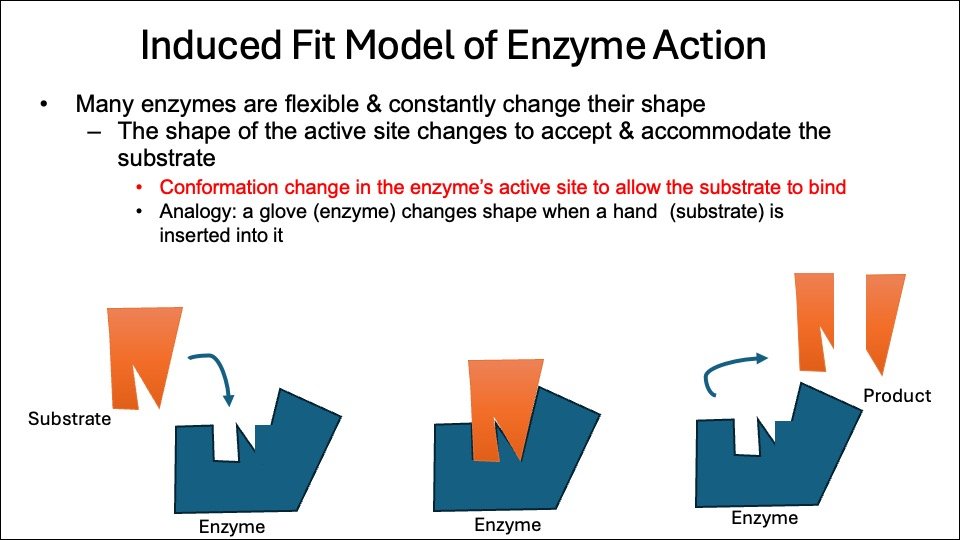
Figure: Induced fit Model of enzyme- substrate interactions
Mechanism of Enzyme Action
In 1913, Michaelis-Menten kinetics, established by Leonor Michaelis and Maud Menten, proposed a credible model for enzyme kinetics. Their model suggests that an enzyme molecule (E) initially combines with a substrate molecule (S) to form an intermediate enzyme-substrate complex (ES). This complex subsequently breaks down to yield the product (P) and releases the enzyme (E). The enzyme, once freed, can then bind to another substrate molecule and repeat the process to produce more product.
Michaelis-Menten kinetics, represents the most fundamental model of enzyme kinetics, which is utilized for enzyme-catalyzed reactions involving a single substrate yielding one product. Here’s how it’s derived:
- Basic Reaction Scheme: The enzyme (E) binds to the substrate (S) to form an enzyme-substrate complex (ES), which then breaks down to form the product (P) and regenerate the enzyme:
E+Sk−1⇌k1ES→k2E+P
- Assumptions:
- Steady-State Assumption: The formation and breakdown of the ES complex reach a steady state, meaning the concentration of ES remains constant over time.
- Initial Rate Assumption: The initial rate of reaction is measured, so the concentration of product (P) is negligible, and the reverse reaction (P to ES) can be ignored.
2.Rate of Formation and Breakdown of ES: The rate of formation of ES is given by:
d[ES]/dt=k1[E][S]−k−1[ES]−k2[ES]
3.At steady state, d[ES]/dt=0, so:
k1[E][S]=(k−1+k2)[ES]
4.Michaelis Constant (Km): Define the Michaelis constant, (Km), as:
Km=k−1+k2/k1
5.Solving for [ES]: Rearrange the steady-state equation to solve for [ES]:
[ES]=[E][S]/Km
6.Total Enzyme Concentration: The total enzyme concentration, [E]0, is the sum of free enzyme [E] and enzyme-substrate complex [ES]:
[E]0=[E]+[ES]
Substitute [ES] from the previous step:
[E]0=[E]+[E][S]/Km
Solve for [E]:
[E]=[E]0/1+[S]/Km
7.Rate of Product Formation (V): The rate of product formation is given by:
V=k2[ES]
Substitute [ES] from step 5:
V=k2[E][S]/Km
Substitute [E] from step 6:
V=k2.[E]0[S]/1+[S]/Km
Simplify to get the Michaelis-Menten equation:
V=Vmax[S]/Km+[S]
where (Vmax = k2[E]0)
This equation describes how the reaction rate (V) depends on the substrate concentration [S] and the enzyme’s kinetics parameters Vmax and Km.
ENZYMES ENHANCE THE RATE OF REACTION BY LOWERING FREE
ENERGY OF ACTIVATION
A chemical reaction from substrate (S) to product (P) occurs when a sufficient number of S molecules simultaneously acquire enough energy to reach an activated condition known as the “transition state.” In this state, the likelihood of forming or breaking chemical bonds to produce P is significantly elevated.
The transition state is the top of the energy barrier separating the reactants and products. The rate of a given reaction will vary directly as the number of reactant molecules in the transition state. The “energy of activation is the amount of energy required to bring all the molecules in 1 gram-mole of a substrate at a given temperate to the transition state.
A rise in temperature, by increasing thermal motion and energy, causes an increase in the number of molecules on the transition state and thus accelerates a chemical reaction. Addition of an enzyme or any catalyst can also bring about such acceleration.
The enzyme combines transiently with the substrate to produce a transient state having lower energy of activation than that of substrate alone. This results in acceleration of the reaction. Once the products are formed, the enzyme (or biocatalyst) is free or regenerated to combine with another molecule of the substrate and repeat the process.
Activation energy is a term introduced in 1889 by the Swedish scientist Syante Arrhenius to describe as Activation energy is the minimum amount of energy necessary for a chemical reaction to move forward. The activation energy is the energy difference between reactant and transition state.
During the formation of an ES complex, the substrate attaches itself to the specific active sites on the enzyme molecule by Reversible interactions formed by Electrostatic bonds, Hydrogen bonds, Vander Waals forces, Hydrophobic interactions.
Factors Affecting Enzyme Activity
Physical and chemical factors are affecting the enzyme activity. These include
A. Temperature
B. pH
C. Substrate
D. Enzyme concentration etc.
A. Temperature
Temperature has a significant impact on enzyme activity.
- Optimal Temperature: Each enzyme has an optimal temperature at which it functions most efficiently. For many human enzymes, this is around 37°C (98.6°F), which is the normal body temperature.
- Increased Activity: As the temperature approaches the optimal level, enzyme activity tends to increase. Higher temperatures contribute to greater kinetic energy, which leads to more frequent interactions between enzymes and substrates.
- Denaturation: Above the optimal temperature, the enzyme’s structure begins to deteriorate, a phenomenon referred to as denaturation. Consequently, the active site alters its shape, inhibiting the enzyme’s ability to bind efficiently to its substrate.
- Decreased Activity: At temperatures above the optimal range, enzyme activity decreases sharply. Denaturation can be irreversible, leading to a permanent loss of enzyme function.
- Low Temperatures: At reduced temperatures, the activity of enzymes decreases due to the slower molecular movements, leading to less frequent collisions between enzymes and their substrates.
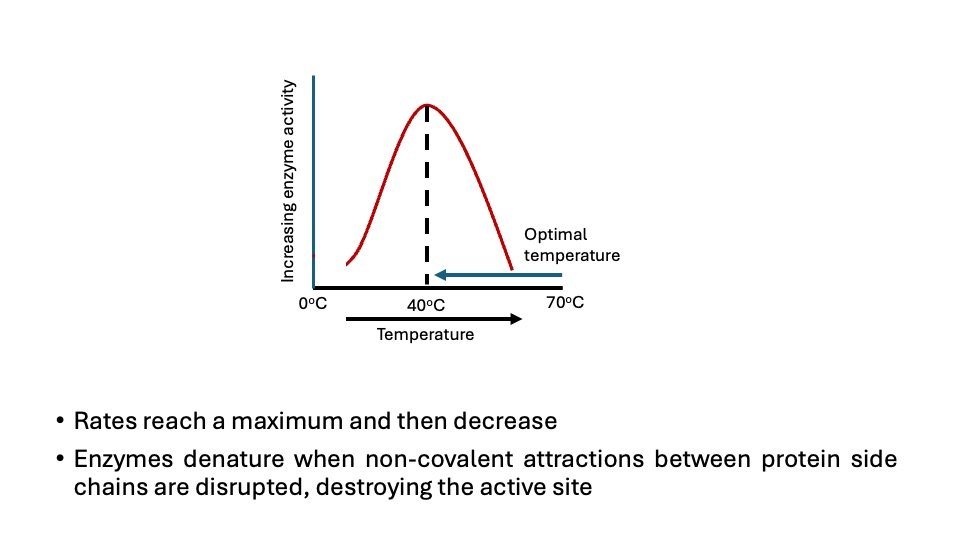
Figure. Effect of temperature on enzymatic reaction
B. Effect of pH
The concentration of H+ ions influences the velocity of reactions in multiple ways. Primarily, the catalytic activity often necessitates that both the enzyme and substrate possess certain chemical groups in either an ionized or a non-ionized state for effective interaction.
For example, Catalytic activity may require that an amino-group of the enzyme be in the protonated form (-NH3+). At alkaline pH this group is deprotonated, and the rate of reaction therefore declines.
Extreme pH can also lead to denaturation of the enzyme because the structure of the catalytically active protein molecule depends on the ionic character of the amino acid chains.
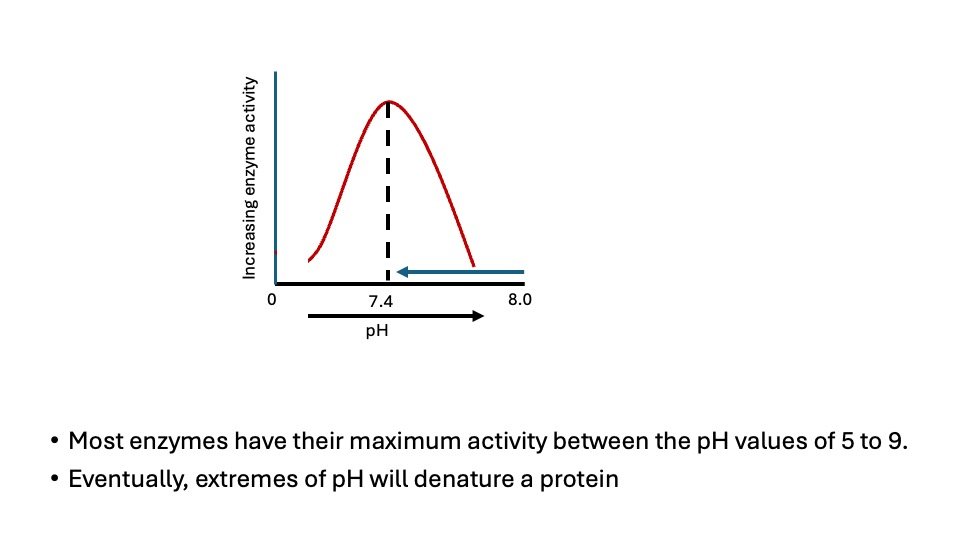
Figure. Effect of pH on enzymatic reaction
The pH at which maximum enzyme activity is achieved is different for different enzymes, and it reflects the pH at which the enzyme functions in the body. For example, pepsin, a digestive enzyme in the stomach, has maximum action at pH 2, whereas other enzymes, designed to work at neutral pH, are denatured by such an acidic environment.
C. Concentration of substrate
Increased substrate concentration influences enzyme activity at constant enzyme concentration like pH, and temperature.
The enzyme activity increases as the substrate concentration increases until a maximum velocity is achieved. Further, an increase in substrate concentration has no effect on the rate of the reaction. This condition shows that as concentration of substrate is increased, the substrate molecule combine with all available enzyme molecules at their active site till not more active sites are available (The active Sites become saturated). At this state, the enzyme is obtained it maximum rate (V max).
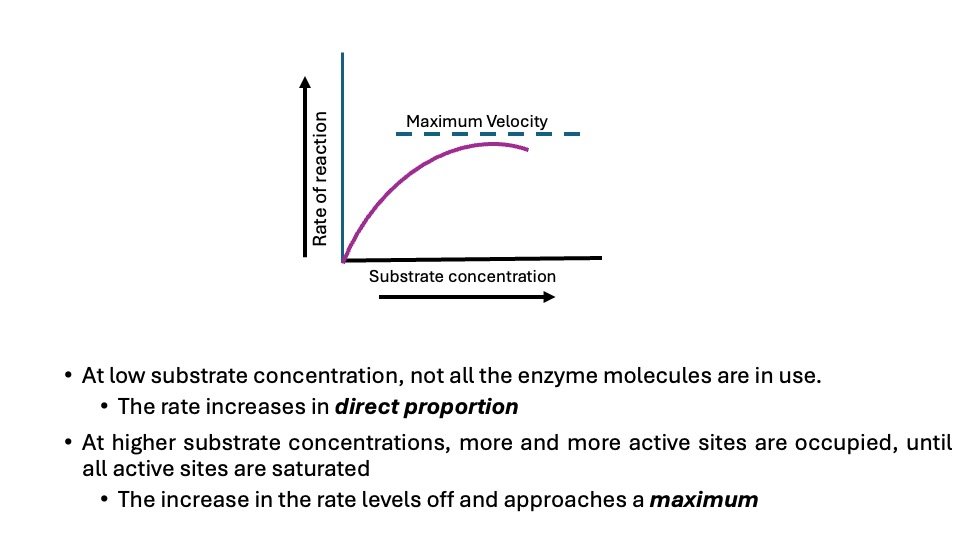
Figure: Effect of concentration of substrate on enzyme activity
D. Enzyme Concentration
At all substrate concentrations, the reaction rate is linearly proportional to the enzyme concentration. For instance, if the concentration of the enzyme is halved, the initial rate of the reaction (Vo) is lowered to half of its original value.
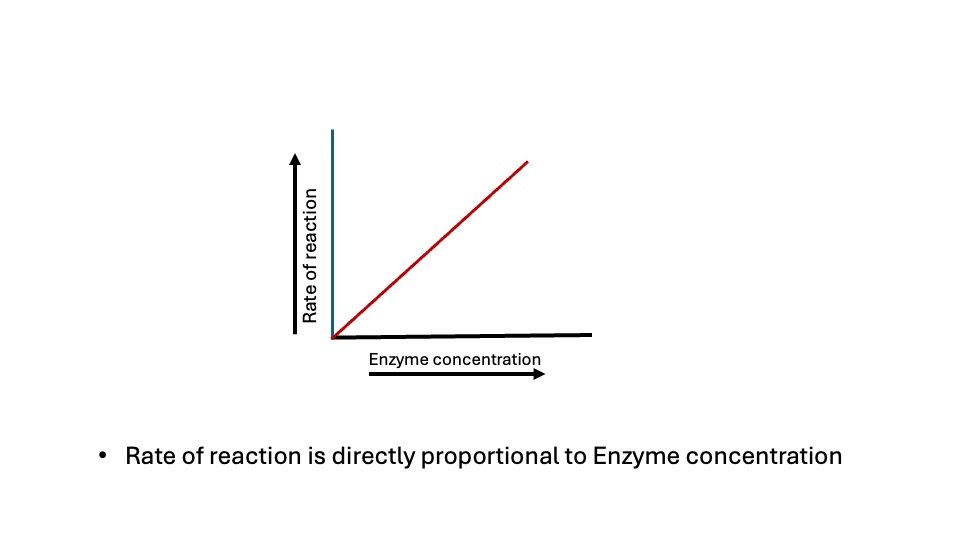
Figure: Effect of Concentration of Enzyme on enzyme activity
The characteristic shape of the substrate saturation curve for an enzyme can be expressed mathematically by the Michaelis Menten equation:
Figure: Relationship between [S] and Km
Where: V= Velocity at a given concentration of substrate (initial reaction velocity)
Vmax = Maximal velocity possible with excess of substrate
[S] = concentration of the substrate at velocity V
Km = Michaelis-constant of the enzyme for particular substrate (Vary with substrate).
Km represents the relationship between substrate concentration and enzyme-catalyzed reaction velocity.Take the point in which 50% of the active site of the enzyme will be saturated by substrate, assume that at ½ Vmax-50% of the active site of enzyme becomes saturated. Therefore:
Vo = ½ Vmax, at 50% saturation
½ Vmax = Vmax[S]
Km + [S] 2[S] = Km+ [S]
Km= [S]
Significance of Km and Vmax
Understanding Km and Vmax (Michaelis-Menten kinetics) are critical to working with enzyme inhibitors.
Km Kinetics
The Michaelis-Menten constant, Km, defined as the substrate concentration at which half maximum velocity is observed. It varies considerably from enzyme to enzyme. It also varies with different substrates for the same enzyme. When the substrate concentration is equal to the Km value, half of the enzyme’s active sites are occupied by substrate molecules.
Key Points About Km:
- Km is a constant with units M
- Km is a constant derived from rate constants
- Km estimates the dissociation constant of enzyme-substrate interactions under true Michaelis-Menten conditions.
- A small Km means tight binding; a high Km means weak binding
In the Michaelis-Menten model for enzyme kinetics, it is assumed that the enzyme first reacts with the substrate to form an enzyme-substrate complex (ES) that breaks down to form product (P) and free enzyme (E). Hence, Km can be expressed in terms of three rate constants (k1, k-1, and k2).
Km– can be defined as the substrate concentration at which an enzyme produces half of its maximum amount of product. Velocity (i.e Km is numerically equal to the substrate concentration of which the reaction velocity equal to ½ Vmax).
Km- is characteristic of an enzyme and a particular substrate, It reflects the affinity of the enzyme for that substrate.
Km- values varies from enzyme to enzyme and used to characterized different enzymes.
Km- values of an enzyme helps to understand the nature and speed of the enzyme catalysis.
Small Km – A numerically small (Low) km reflects a high affinity of the enzyme for substrate because a low conc of substrate is needed to half saturate the enzyme- that is reach a velocity of ½ Vmax.
High Km – A numerically large (high) Km reflects a low affinity of enzyme for substrate b/c a high conc of substrate is needed to half saturate the enzyme. High Km Value for an enzyme means the catalysis of that enzyme is slow compared to low Km. Km does not vary with the concentration of enzyme.
Order of Reaction
When [S] substrate concentration is much less than Km, the velocity of the reaction is roughly proportional to the substrate concentration. The rate of reaction is then said to be first order configuration with respect to substrate. When [S] is much greater than Km, the velocity is constant and equal to V max. The rate of reaction is then independent of substrate concentration and said to be zero order with respect to substrate concentration.
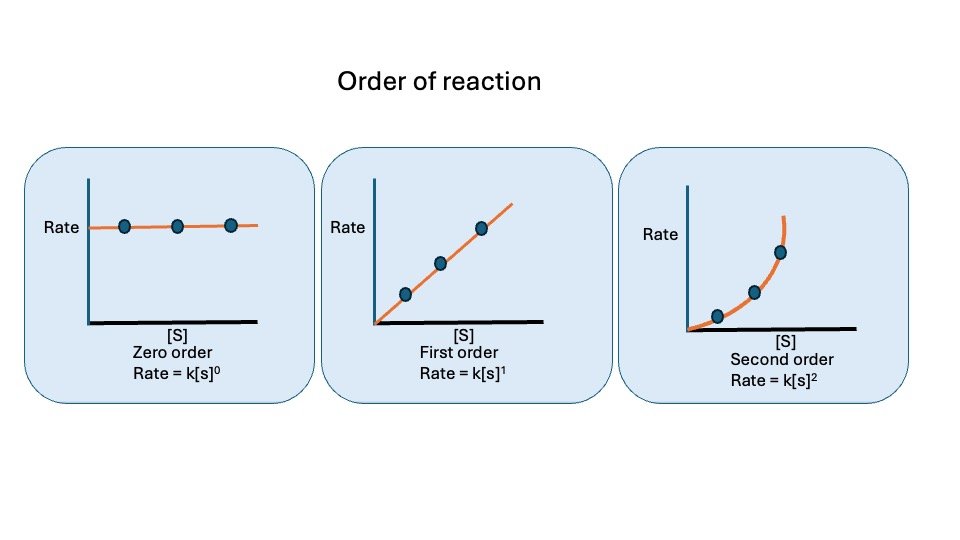
Figure : Order of reaction
Reference book
- Harper’s Illustrated Biochemistry, Thirty-Second Edition
- Textbook of Biochemistry with Clinical Correlations Hardcover – Illustrated, 22 January 2010 by Thomas M. Devlin
- Lehninger Principles of Biochemistry: 6th Edition
- Lippincott’s Illustrated Reviews – Biochemistry South