Cholesterol is a hydrophobic molecule containing 27-carbon compounds with 4 rings (A, B, C, D) & a side chain. A single hydroxyl group at carbon 3 of the A ring, to which a fatty acid can be linked to generate an even more hydrophobic cholesteryl ester.
Cholesterol is synthesized by nearly all human tissues, However, the most cholesterol is synthesized in the cytoplasm of liver, intestines, adrenal cortex, and reproductive tissues. Acetyl coenzyme A (CoA) provides all the carbon atoms in cholesterol, and nicotinamide adenine dinucleotide phosphate provides the reducing equivalents. The pathway is driven by hydrolysis of the high-energy thioester bond of acetyl CoA and the terminal phosphate bond of adenosine triphosphate. The rate-limiting and regulated step in cholesterol synthesis is catalyzed by the smooth endoplasmic reticulum–membrane bound protein, hydroxymethylglutaryl coenzyme A (HMG CoA) reductase, which produces mevalonate from HMG CoA.
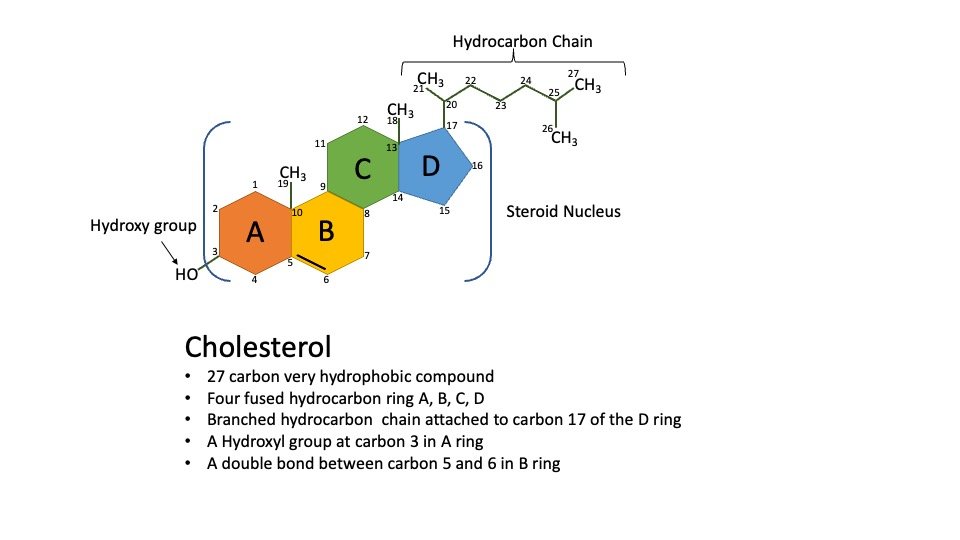
Figure: Structure of Cholesterol.
Biological significance of cholesterol
1) Cholesterol is an essential lipid constituent of cell membranes. Level of Cholesterol in the membrane modulates physical properties of these membranes that in turn affect the function of membrane bound proteins such as receptors and transporters. Depletion of membrane cholesterol cripples many cellular functions.
2) Cholesterol is the biosynthetic precursor of bile salts, which are essential for lipid digestion and absorption.
3) Intermediates of cholesterol biosynthesis are required to make vitamin D3.
4) It is posttranslational modification of membrane proteins like dolichol, ubiquinone.
5) High plasma cholesterol promotes atherosclerosis.
6) Cholesterol is the precursor of all steroid hormones, namely, androgens, estrogens, progestins, glucocorticoids, and mineralocorticoids.
7) It is the component of lipoproteins to lipids transport in the body.
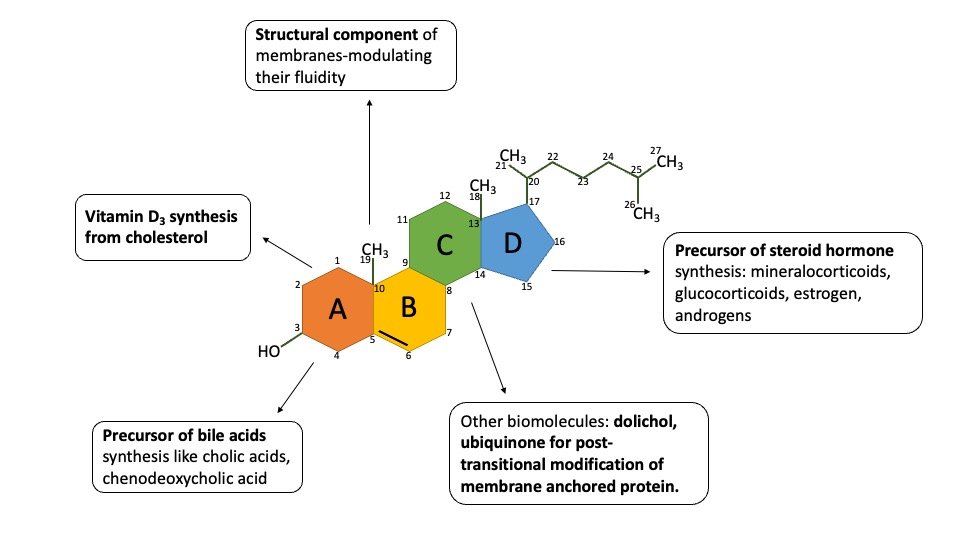
Salient feature of cholesterol:
- Cholesterol is present in tissues and plasma either as free cholesterol or as cholesteryl ester (combined with a long-chain fatty acid)
- Cholesterol is an amphipathic lipid – essential structural component of membranes, important for the maintenance of the correct permeability and fluidity, and it also forms the outer layer of plasma lipoproteins
- Cholesterol occurs in foods of animal origin such as egg yolk, meat, liver and brain
- Plasma LDL is the vehicle that supplies cholesterol and cholesteryl ester to many tissues
- Plasma HDL removes free cholesterol from tissues andtransported to the liver, (reverse cholesterol transport) where it is eliminated from the body either unchanged or after conversion to bile acids
Cholesterol Biosynthesis:
Virtually all nucleated cells are capable of cholesterol synthesis, which occurs in the cytosolic and the endoplasmic reticulum compartments. A relatively constant level of cholesterol in the blood (150–200 mg/dL) is maintained primarily by controlling the level of de novo synthesis. The level of cholesterol synthesis is regulated in part by the dietary intake of cholesterol. The greatest proportion of cholesterol is used in bile acid synthesis.
Cholesterol biosynthesis starts in cytosol and further modification occurs in the endoplasmic reticulum of Liver and intestine. Liver is the major site for cholesterol synthesized in the body. The synthesis follows five major steps which include:
a) Acetyl CoA(2C) is converted to HMG-CoA.
b) HMG CoA is reduced to Mevalonate by a reductase.
c) Mevalonate undergoes three times Phosphorylation, in the presence of 3 ATPs and various kinases. The product is 3-phosphor-5 pyrophosphate mevalonate.
d) Dephosphorylation, decarboxylation converts it to Isopentenyl pyrophosphate.
e) It is isomerised to dimethylallyl pyrophosphate by isomerase.
f) Isopentenyl pyrophosphate and dimethylallyl pyrophosphate form Geranyl PP (10C).
g) Geranyl PP and one more molecule of Isopentenyl PP → Farnesyl PP (15C).
h) Two of Farnesyl PP join to form Squalene (30C).
Squalene undergoes cyclization, loses three carbon atoms, acquires a double bond, and forms cholesterol.
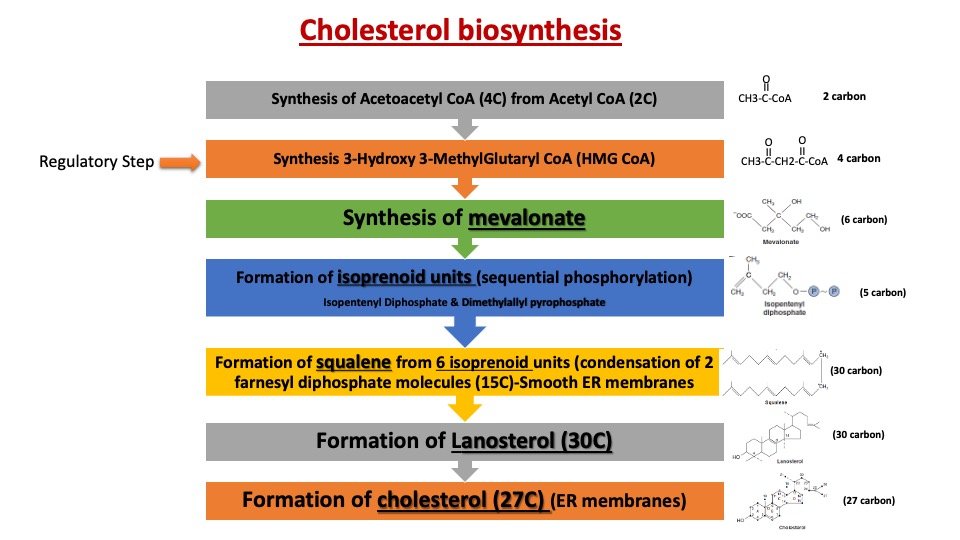
Different steps of cholesterol biosynthesis:
| Step | Description | Enzyme |
| 1 | Synthesis of mevalonate from acetyl-CoA | HMG-CoA reductase |
| 2 | Conversion of mevalonate to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) | Mevalonate kinase, phosphomevalonate kinase, and mevalonate diphosphate decarboxylase |
| 3 | Formation of geranyl pyrophosphate (GPP) from IPP and DMAPP | Geranyl transferase |
| 4 | Formation of farnesyl pyrophosphate (FPP) from GPP | Farnesyl transferase |
| 5 | Formation of squalene from FPP | Squalene synthase |
| 6 | Formation of lanosterol from squalene | Lanosterol synthase |
| 7 | Conversion of lanosterol to cholesterol | Multiple enzymes, including 7-dehydrocholesterol reductase and 27-hydroxylase |
Regulation of cholesterol biosynthesis:
Highly regulated at multiple levels:
- Feed-back inhibition from cholesterol
- Synthesis of HMG CoA reductase enzyme (at transcriptional level)
- Rate of translation of HMG CoA reductase (at translational level)
- Degradation of the HMG CoA reductase: The degradation of enzyme by ubiquitination and proteasome dependent manner under the influence of cholesterol.
- Post-translational modification i.e. phosphorylation of HMG CoA reductase regulates its activity to catalyze the reaction.
Clinical Notes:
A) Increased by insulin, thyroid hormones (HMG CoA Dephosphorylation)
B) Decreased by glucagon, glucocorticoids (HMG CoA phosphorylation)
HMG CoA reductase is the regulatory enzyme:
Inhibition-
1. Dietary cholesterol inhibits endogenous synthesis.
2. Fasting leads to low levels of the key enzyme.
3. Insulin activates protein phosphatase which converts it to an active enzyme. Glucagon decreases its activity through cAMP dependent protein kinase.
4. Whenever ATP levels are low, the enzyme is switched off by AMP activated protein kinase.
5. mRNA for HMG CoA reductase is under the control of sterols. High concentration of sterols inhibits the synthesis of mRNA, thereby the synthesis of enzymes.
Factor which influences the activity of HMG-CoA Reductase:
| S.No. | Factor | Effect on HMG-CoA Reductase Activity |
| 1. | Insulin | Stimulates the activity of HMG-CoA reductase, increasing cholesterol synthesis |
| 2. | Glucagon | Inhibits the activity of HMG-CoA reductase, decreasing cholesterol synthesis |
| 3. | SREBP | Activates the expression of HMG-CoA reductase, promoting cholesterol synthesis |
| 4. | LDL Receptors | High levels of LDL receptors decrease the activity of HMG-CoA reductase, while low levels increase its activity |
| 5. | Statins | Inhibit the activity of HMG-CoA reductase, reducing cholesterol synthesis |
| 6. | Dietary Cholesterol | High dietary intake of cholesterol decreases the activity of HMG-CoA reductase, while low intake increases its activity |
| 7. | Omega-3 Fatty Acids | Inhibit the activity of HMG-CoA reductase, reducing cholesterol synthesis |
| 8. | Exercise | Increases the expression of LDL receptors and enhances the clearance of LDL cholesterol, reducing cholesterol synthesis |
Regulation of Cholesterol biosynthesis based on long and short term:
Cholesterol biosynthesis is an extremely complex process. HMG-CoA Reductase is the rate-determining step on the pathway for synthesis of cholesterol and a major control point.
(A) long-term regulation of cholesterol synthesis: Transcriptional regulation of cholesterol synthesis starts in the endoplasmic reticulum.
(1) Regulate the formation of HMG-CoA Reductase and other enzymes involved in the biosynthesis of cholesterol.
Transcriptional regulation: a family of transcription factors designated SREBP (Sterol Regulatory Element Binding Proteins) regulate synthesis of cholesterol and fatty acids
i) SREBP-2 mainly regulates cholesterol synthesis.
ii) SREBP-1 mainly regulates fatty acid synthesis.
When sterol level is low, SREBP-2 is released by cleavage of a membrane-bound precursor protein, SREBP-2 activates transcription of genes for HMG-CoA Reductase and other enzymes of the pathway for cholesterol synthesis activated SREBPs enter the nucleus and turn on the expression of genes that contain sterol regulatory element (SRE) elements in their promoters, such as the low-density lipoprotein receptor (LDLR), HMG-CoA synthase, squalene synthase and fatty acid synthase
(2) Regulation of HMG-CoA Reductase degradation (proteolysis) Proteolysis of HMG-CoA Reductase is stimulated by Cholesterol level, oxidized derivatives of Cholesterol, mevalonate, and farnesol. HMG-CoA Reductase includes a transmembrane sterol-sensing domain that has a role in activating degradation of the enzyme via the proteasome dependent.
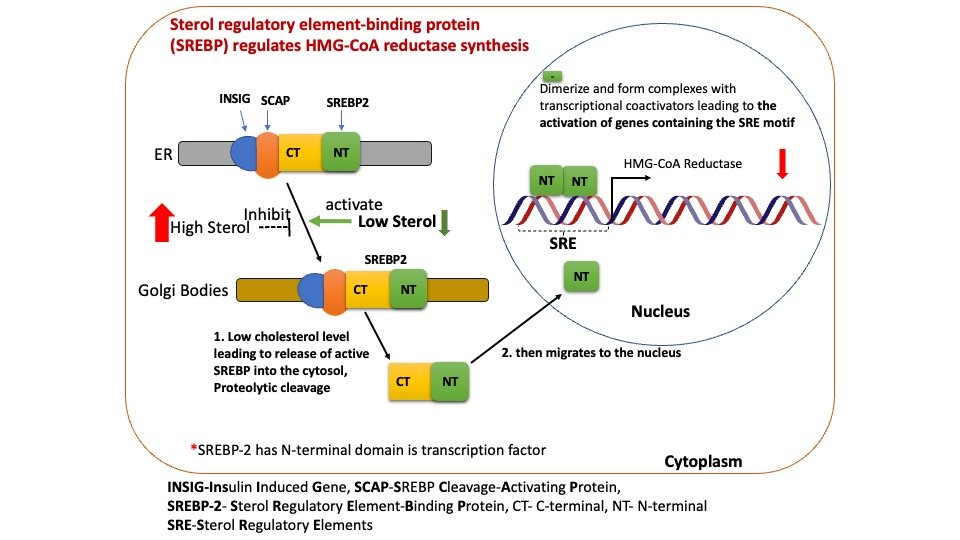
Figure: Long term regulation of cholesterol synthesis through gene activation.
(B) Short-term regulation
HMG-CoA Reductase is inhibited by phosphorylation, catalyzed by AMP-dependent Protein Kinase-AMPK (which also regulates FA synthesis and catabolism). AMPK kinase is active when cellular AMP is high, corresponding to when ATP is low thus, when cellular ATP is low, energy is not expended in synthesizing cholesterol.
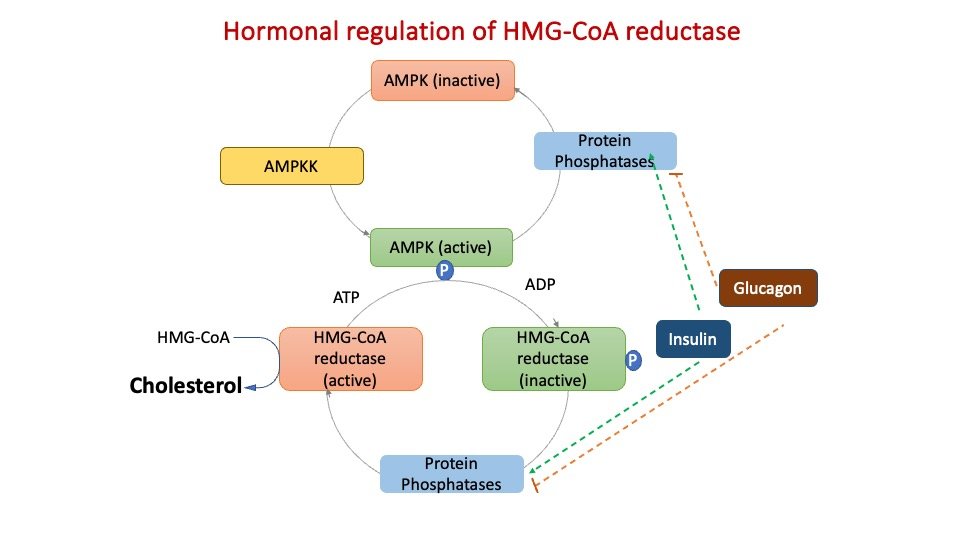
Figure: Short term regulation of cholesterol synthesis through phosphorylation.
Mechanism of Sterol regulatory element-binding protein (SREBP) regulates HMG-CoA reductase synthesis:
SREBPs are membrane-bound (ER) transcription factors that regulate the transcription of genes involved in the cellular uptake and metabolism of cholesterol and other lipids.
There are three isoforms of SREBPs:
(1 and 2) SREBP-1a and -1c, resulting from alternative splicing of the SREBP1 gene and preferentially activating genes in fatty acid synthesis.
(3) SREBP-2, primarily activating/regulating genes in the cholesterol metabolism pathway including enzymes of cholesterol biosynthesis, the LDL receptor, and mediators of cholesterol efflux.
SREBP activates its target genes by binding to sterol regulatory elements (SREs) in their promoters. SREBP-2 is the predominant isoform affecting cholesterol homeostasis through the regulation of the expression of 3-hydroxy-3-methylglutaryl coenzyme A synthase (HMG-CoAS), HMG-CoA reductase, farnesyl diphosphate synthase, and squalene synthase. The molecular switch that controls sterol metabolism in animal cells is a simple hexapeptide targeting signal (MELADL) in a single membrane protein (Scap). When cells have sufficient sterol content SREBP and SCAP are retained in the ER. SCAP binds cholesterol which promotes the interaction with Insig and the entire complex is retained in the ER.
1) When cholesterol levels are low, the transcription factor, sterol regulatory element–binding protein-2 (SREBP-2), attached to a sterol regulatory element (SRE), activates the expression of the reductase gene, resulting in increased enzyme and, consequently, cholesterol production.
2) When cholesterol levels are high, the rate of reductase degradation increases.
3) Adenosine monophosphate (AMP)–activated protein kinase [AMPK], which phosphorylates and inactivates the reductase, and an insulin-activated protein phosphatase regulate reductase activity (which dephosphorylates and activates it).
4) Insulin upregulates the expression of the reductase gene, whereas glucagon downregulates its expression.
5) Statins are competitive HMG CoA reductase inhibitors. These medications are prescribed to hypercholesterolemic patients to reduce plasma cholesterol levels.
(C) Pharmacological–
Hyperlipidemia drugs – competitive inhibitors of HMG-CoA Reductase (statins)
| Drug Class | Mechanism of Action | Examples |
| Statins | Inhibit HMG-CoA reductase enzyme | Atorvastatin, Simvastatin, Rosuvastatin |
| Ezetimibe | Inhibits cholesterol absorption | Ezetimibe |
| PCSK9 inhibitors | Inhibit PCSK9 protein which promotes LDL receptor degradation | Alirocumab, Evolocumab |
| Bile acid sequestrants | Bind to bile acids in the intestine, preventing reabsorption | Cholestyramine, Colesevelam |
| Fibrates | Activate PPARα receptor, increasing lipoprotein lipase activity and reducing VLDL levels | Fenofibrate, Gemfibrozil |
| Niacin | Reduces VLDL and LDL levels, increases HDL levels | Niacin |
Clinical Note:
Role of lipoprotein(a) or Lp(a)
Lipoprotein (a) is a tiny particle with a structure substantially identical to an LDL particle. a high plasma concentration is associated with an elevated coronary heart disease risk. It is distinguished by the presence of an extra apolipoprotein molecule, apo(a), covalently attached to apo B-100 at a single location. Trans fatty acids have been demonstrated to elevate Lp(a), whereas ω-3 fatty acids lower it. Diet may therefore play a role.
Clinical Note: Lp(a) is structurally homologous to plasminogen, the precursor of a blood protease whose target is fibrin, the main protein component of blood clots. It is hypothesized that elevated Lp(a) slows the breakdown of blood clots that trigger heart attacks because it competes with plasminogen for binding to fibrin. The physiologic function of Lp(a) is unknown. Niacin reduces Lp(a), as well as LDL-cholesterol and TAGs, and raises HDL.
Catabolism of Cholesterol:
Intestinal Bacteria converts cholesterol to coprostanol which is excreted in feces. Cholesterol breaks down to cholic acid and chenodeoxycholic acid. Both are bile acids. They combine with sodium, Potassium to form bile salts. The key enzyme alpha-hydroxylase is inhibited by high concentration of bile acids.
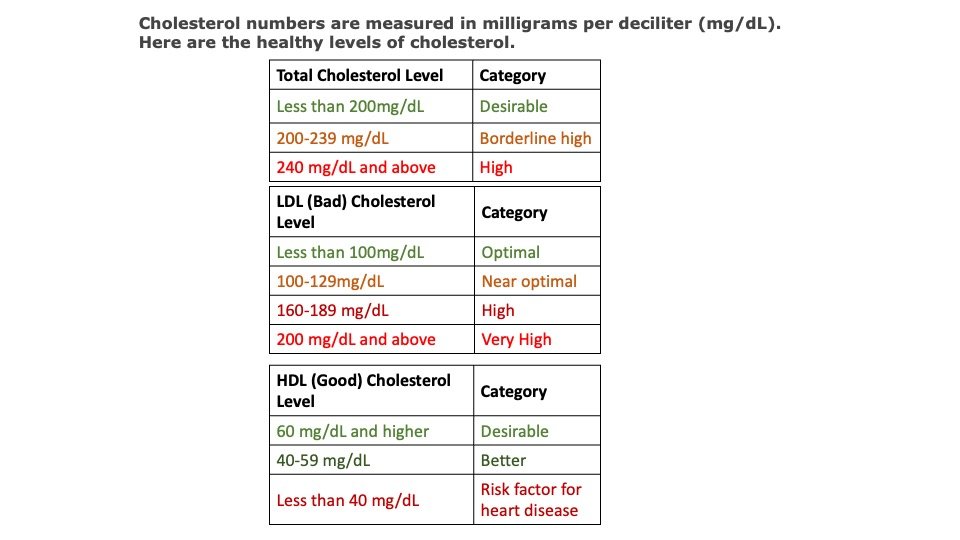
Laboratory: Estimation of Cholesterol
Cholesterol can be estimated in the laboratory using various methods, such as enzymatic methods, colorimetric methods, and high-performance liquid chromatography (HPLC) methods.
A) Enzymatic Method:
The patient’s blood sample needs to be centrifuged to separate serum from blood cells. To a serum sample, add a cholesterol esterase/oxidase reagent. These enzymes metabolize cholesterol esters into cholest-4-en-3-one and hydrogen peroxide from free cholesterol. Add a second reagent containing peroxidase and 4-aminoantipyrine. Peroxidase reacts with hydrogen peroxide to generate a colored molecule that can be detected spectrophotometrically at a particular wavelength. Using a standard curve, the cholesterol levels in the serum sample can be compared to the color developed.
B) Colorimetric Method:
The blood sample of the patient needs to be centrifuged to separate serum from blood cells. Add a color-producing detergent reagent, such as ferric chloride, to the serum sample. The discharge of cholesterol from lipoprotein particles by detergent. Ferric chloride and released cholesterol produce a spectrophotometrically detectable colored complex at a particular wavelength. Using a standard curve, the cholesterol levels in the serum sample can be compared to the color developed.
*Please be advised that while we have taken every effort to assure the accuracy of this information.Please consult your personal physician for more details and clarification.
Reference Books:
Lippincott Illustrated Reviews: Biochemistry
Multiple choice questions
1) Which enzyme is responsible for the rate-limiting step in cholesterol biosynthesis?
A. Acetyl-CoA carboxylase
B. HMG-CoA reductase
C. Squalene synthase
D. Lanosterol synthase
Answer:
2) Which of the following factors stimulates the activity of HMG-CoA reductase?
A. Insulin
B. Glucagon
C. SREBP
D. LDL receptors
Answer:
3) Which of the following is a common class of drugs that inhibit HMG-CoA reductase?
A. Statins
B. Fibrates
C. Niacin
D. Ezetimibe
Answer:
4) Which of the following is a product of the mevalonate pathway?
A. Isopentenyl pyrophosphate (IPP)
B. Dimethylallyl pyrophosphate (DMAPP)
C. Geranyl pyrophosphate (GPP)
D. Squalene
Answer:
5) Which of the following enzymes is responsible for the conversion of lanosterol to cholesterol?
A. 7-dehydrocholesterol reductase
B. 27-hydroxylase
C. HMG-CoA reductase
D. Lanosterol synthase
Answer:
6) Which of the following is a transcription factor that activates the expression of HMG-CoA reductase?
A. Insulin
B. Glucagon
C. SREBP
D. LDL receptors
Answer:
7) Which of the following factors inhibits the activity of HMG-CoA reductase?
A. Insulin
B. Glucagon
C. SREBP
D. LDL receptors
Answer:
8) Which of the following dietary factors can increase the activity of HMG-CoA reductase?
A. High intake of dietary cholesterol
B. High intake of omega-3 fatty acids
C. Low intake of dietary fiber
D. High intake of trans fats
Answer:
9) Which of the following is a factor that can increase the expression of LDL receptors?
A. Insulin
B. Glucagon
C. SREBP
D. Statins
Answer:
10) Which of the following is a factor that can increase the expression of LDL receptors?
a. Insulin
b. Glucagon
c. SREBP
d. Statins
Answer:
11) Which enzyme is responsible for the rate-limiting step of cholesterol synthesis?
A. HMG-CoA reductase
B. Acetyl-CoA carboxylase
C. Fatty acid synthase
D. AMPK
Answer:
12) What is the primary site of cholesterol synthesis in the body?
A. Liver
B. Adipose tissue
C. Intestine
D. Kidneys
Answer:
13) Which lipoprotein is responsible for transporting cholesterol from peripheral tissues back to the liver?
A. LDL
B. HDL
C. VLDL
D. Chylomicrons
Answer:
14) Which enzyme is responsible for breaking down LDL particles in the liver?
A. LCAT
B. CETP
C. HL
D. LDLR
Answer:
15) Which of the following is an example of a genetic disorder that can result in high cholesterol levels?
A. Type 2 diabetes
B. Hypothyroidism
C. Familial hypercholesterolemia
D. Chronic liver disease
Answer:
16) Which of the following is a class of drugs that can be used to lower cholesterol levels?
A. Anticoagulants
B. ACE inhibitors
C. Beta blockers
D. Statins
Answer:
17) Which of the following is a lifestyle modification that can help lower cholesterol levels?
A. Smoking cessation
B. Increased alcohol consumption
C. Sedentary behavior
D. High sugar intake
Answer:
18) Which lipoprotein is responsible for transporting newly absorbed dietary cholesterol from the intestine to the liver?
A. LDL
B. HDL
C. VLDL
D. Chylomicrons
Answer:
19) Which of the following is a dietary factor that can contribute to high cholesterol levels?
A. High fiber intake
B. Low sodium intake
C. High saturated fat intake
D. Low carbohydrate intake
Answer:
MCQs based on assertion reasoning:
1. Assertion: Hypercholesterolemia is a medical condition characterized by high levels of cholesterol in the blood.
Reasoning: Hypercholesterolemia can be caused by genetic factors, such as mutations in the LDL receptor gene, or lifestyle factors, such as a diet high in saturated fats.
A. Both Assertion and Reasoning are true and Reasoning is the correct explanation of Assertion.
B. Both Assertion and Reasoning are true but Reasoning is NOT the correct explanation of Assertion.
C. Assertion is true but Reasoning is false.
D. Assertion is false but Reasoning is true.
2. Assertion: The liver is the primary site of cholesterol synthesis in the body.
Reasoning: Cholesterol synthesis occurs in many tissues, but the liver is responsible for most of the body’s cholesterol production.
A. Both Assertion and Reasoning are true and Reasoning is the correct explanation of Assertion.
B. Both Assertion and Reasoning are true but Reasoning is NOT the correct explanation of Assertion.
C. Assertion is true but Reasoning is false.
D. Assertion is false but Reasoning is true.
3. Assertion: High levels of LDL cholesterol are a risk factor for cardiovascular disease.
Reasoning: LDL cholesterol can build up in the walls of arteries, leading to the formation of plaques that can cause atherosclerosis.
A. Both Assertion and Reasoning are true and Reasoning is the correct explanation of Assertion.
B. Both Assertion and Reasoning are true but Reasoning is NOT the correct explanation of Assertion.
C. Assertion is true but Reasoning is false.
D. Assertion is false but Reasoning is true.
4. Assertion: Statins are a class of drugs used to lower cholesterol levels.
Reasoning: Statins work by inhibiting the enzyme HMG-CoA reductase, which is responsible for the rate-limiting step of cholesterol synthesis.
A. Both Assertion and Reasoning are true and Reasoning is NOT the correct explanation of Assertion.
B. Both Assertion and Reasoning are true but Reasoning is the correct explanation of Assertion.
C. Assertion is true but Reasoning is false.
D. Assertion is false but Reasoning is true.
5. Assertion: Familial hypercholesterolemia is a genetic disorder that can cause high cholesterol levels.
Reasoning: Familial hypercholesterolemia is caused by mutations in the LDL receptor gene, which leads to impaired clearance of LDL cholesterol from the blood.
A. Both Assertion and Reasoning are true and Reasoning is NOT the correct explanation of Assertion.
B. Both Assertion and Reasoning are true but Reasoning is the correct explanation of Assertion.
C. Assertion is true but Reasoning is false.
D. Assertion is false but Reasoning is true.